Langmuir
The most important model of adsorption is the Langmuir isotherm, introduced by I. Langmuir in 1916. This model assumes that:
Molecules adsorb on the surface (but not on each other)
Adsorbed molecules do not interact with each other
The surface is homogeneous: all locations adsorb molecules with the same enthalpy.
Langmuir's original derivation is based on setting the rates of adsorption and desorption equal to each other and solving for the steady-state coverage at the surface, but we can equally well apply simple chemical equilibrium theory to the problem. First we write the adsorption process as a chemical reaction:
where is a surface site, which is then blocked from adsorbing another molecule. The corresponding chemical equilibrium expression is then:
where is the activity of and is the equilibrium constant, and we assume that both the gas and adsorbed film behave ideally. (This is the "no interaction" condition.) We use the fact that the total concentration of surface sites (the monolayer capacity) is a constant, and write:
Finally, defining the surface coverage as , the fraction of surface sites on which are adsorbed molecules of A, we arrive at:
which we can solve for :
which gives the surface coverage as a function of the pressure of gas above the surface, and the equilibrium constant. Several Langmuir isotherms, differing in , are shown in the following plot:
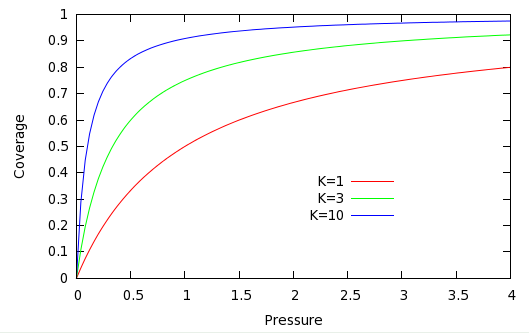
At high , the surface is strongly adsorbent, and the adsorbing gas forms a nearly complete monolayer () at low pressures. At low , much higher gas pressures are required for the same coverage. In all cases, at sufficiently low coverage, the isotherm is linear, that is:
This is called the Henry's Law region, because it is analogous to Henry's Law for gases dissolved in solution.
Finally, the total adsorption is just given by . The Langmuir isotherm is accurate under conditions of low coverage and high adsorption enthalpy (such that interactions between neighboring adsorbed molecules can safely be neglected.) For this reason it is widely used in studies of chemisorption and analysis of heterogeneous catalysis kinetics. Nonetheless, it is also useful for physisorption at high temperatures and low pressures.